» Grenet/Poggerdorf Battery (0639)
Immagini



DESCRIPTION
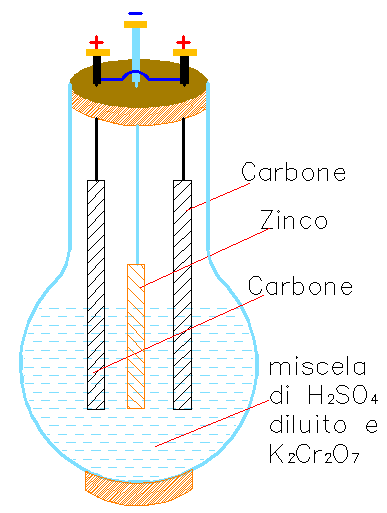
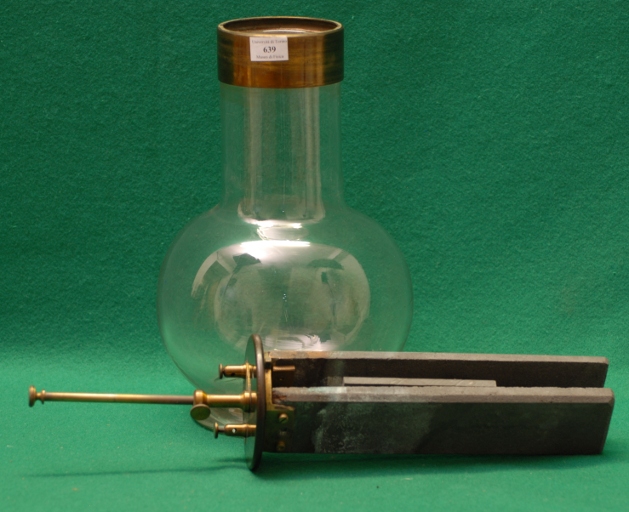
Exhibit 639 is a Grenet battery, based on potassium bichromate, consisting of a spherical glass bottle with a large and long neck and a bakelite tap with three holes. On the inside the positive electrode is made of two carbon strips connected together by a brass wire to their terminals on the tap top. The negative electrode is a zinc strip connected to a metal rod and terminal through the central tap hole (Disegno-1).
The strips are immersed in a dilute acqueous solution of water and sulfuric acid (![Formula: [{\rm H_2 SO_4}] Formula: [{\rm H_2 SO_4}]](/foto/equaz/639_fr01.png) ) and potassium bichromate (
) and potassium bichromate (![Formula: [{\rm K_2 Cr_2 O_7}] Formula: [{\rm K_2 Cr_2 O_7}]](/foto/equaz/639_fr02.png) ). The zinc strip can be raised above the solution when the battery is not in use. The molecules of sulfuric acid dissociate in hydrate ions Hhigh{+} and
). The zinc strip can be raised above the solution when the battery is not in use. The molecules of sulfuric acid dissociate in hydrate ions Hhigh{+} and ![Formula: [{\rm SO_4^{--}}] Formula: [{\rm SO_4^{--}}]](/foto/equaz/639_fr03.png) which transfer their negative charge on the zinc strip forming Zn SO4 . The H+ ions transfer their positive charge on the carbon strips and the liberated hydrogen interacts with the solution forming chromic acid (H2 CrO4).
The chemical reaction is the following:
which transfer their negative charge on the zinc strip forming Zn SO4 . The H+ ions transfer their positive charge on the carbon strips and the liberated hydrogen interacts with the solution forming chromic acid (H2 CrO4).
The chemical reaction is the following:
![Equazione: [{\rm K_2 Cr_2 O_7+7 H_2 SO_4 + H_2 O = 2H_2 CrO_4 + K_2SO_4 + 6H_2 SO_4}] Equazione: [{\rm K_2 Cr_2 O_7+7 H_2 SO_4 + H_2 O = 2H_2 CrO_4 + K_2SO_4 + 6H_2 SO_4}]](/foto/equaz/639_eq01.png)
![Equazione: [{\rm H_2+2H_2 CrO_4 = Cr_2 O_3 + 5 H_2O}] Equazione: [{\rm H_2+2H_2 CrO_4 = Cr_2 O_3 + 5 H_2O}]](/foto/equaz/639_eq02.png)
With the sulfuric acid (![Formula: [{\rm H_2 SO_4}] Formula: [{\rm H_2 SO_4}]](/foto/equaz/639_fr04.png) ) chromic oxide (
) chromic oxide (![Formula: [{\rm Cr_2 O_3}] Formula: [{\rm Cr_2 O_3}]](/foto/equaz/639_fr05.png) ) forms chromatic sulphate, while the potassium sulphate and the chrome form an undesired product, called the allum of chrome.
) forms chromatic sulphate, while the potassium sulphate and the chrome form an undesired product, called the allum of chrome.
![Equazione: [{\rm K Cr_2O_3 + 3H_2SO_4 = Cr_2(SO_4)_3+ 3H_2O}] Equazione: [{\rm K Cr_2O_3 + 3H_2SO_4 = Cr_2(SO_4)_3+ 3H_2O}]](/foto/equaz/639_eq03.png)
![Equazione: [{\rm K_2SO_4 + Cr_2(SO_4)_3 = K_2Cr_2(SO_4)_4 }\quad \text{(Allume of cromo)}] Equazione: [{\rm K_2SO_4 + Cr_2(SO_4)_3 = K_2Cr_2(SO_4)_4 }\quad \text{(Allume of cromo)}]](/foto/equaz/639_eq04.png)
The allum of chrome (potassium chrome sulphate) crystallyzes on the electodes and weakens the battery; in modern batteries one uses chromic acid instead of potassium bichromate.
The battery voltage is about 2 volts, but falls rapidly; for this reason the battery is used for strong currents of short duration. The battery is known with different names: Grenet battery, Poggendorf battery or bichromate battery
Dati Catalografici
| Data di costruzione: | --- |
|---|---|
| Data di carico: | Ignota |
| Nr. Inventario: | Ignoto (Ignoto) |
| Costruttore: | Costruttore sconosciuto |
| Materiale: | vetro, rame, zinco, bakelite |
| Dimensioni: | Altezza complessiva: 36 cm; Diametro massimo: 17 cm; Diametro della base: 13 cm. |
| Conservazione: | buono |
