» Calorimeter (0895)
Immagini


DESCRIPTION
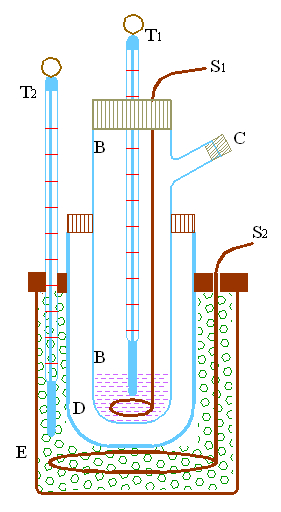
Exhibit 895 is a Beckmann crioscopic calorimeter for determining the freezing temperature of a liquid solution. It consists of a test tube BB (missing) having an entrance tube C on the side and a tap through which passes a thermometer T1 and a stirrer S1 (see design-1). This test tube is inserted in a larger test tube D; the space between these test tubes is filled with air in which occurs the heat exchange between the refrigerant in E and the liquid contained in test tube BB. Both tubes are immersed in the larger glass container E that is filled with the proper refrigerant and stired with the agitator S2. The thermometer T2 shows the temperature of the mixture in E.
Procedure:
The liquid for which the freezing temperature is desired is poured in test tube BB through entrance C that is then closed with a cork. The proper refrigerant (for example, salt and ice) is introduced in E and maintained uniform with the stirrer S2. The apparatus is then watched until the temperature T1 is one or two degrees below the probable freezing point of the liquid. Then the stirrer S1 is suddenly raised and lowered rapidly until solidification occurs. The temperature T1 increases and reaches quickly a constant value, which is the desired freezing point.
This apparatus is used to determine the molecular weight of a substance, utilyzing the lowering of the freezing point of a solution compared to the freezing point of the pure solvent and the experimental observation for which the lowering C of the freezing point of a solution is directly proportional to the molar fraction X of the solute. From this coefficient of proportionality κ = C / X, which for a given solvent is independent of the solute, one can calculate the molecular weight M of the solute with X = P / M, where P is the weight in grams of a substance disolved in 100 grams of solvent. In fact one has
![Equazione: [M = \frac{\kappa P }{C}] Equazione: [M = \frac{\kappa P }{C}]](/foto/equaz/895_eq01.png)
Thus by the simple determination of the freezing point of a solution, we can determine the molecular weight of a substance. (see ref. 2)
For more details on the procedure, see ref. 1. Per maggiori dettagli sul funzionamento dell'apparecchio, vedi ref. 1.
See also exhibit 933
BIBLIOGRAPHY
- [1] BUSSETTI, Giovanni. “Esercitazioni pratiche di fisica". Libreria editrice universitaria Levrotto & Bella, 1965, pp. 341-344
- [2] Ganot's ,"Elementary Treatise on Physics", New York, 1902. Pagg. 330-332.
- [3] Edwin Edser," Heat for Advanced students", London, 1920. pagg. 167-169
- [4] N.Monroe Hopkins,"Experimental Electrochemistry", D. Van Nostrand Company, New York, 1905. Pagg. 32-36
Dati Catalografici
| Data di costruzione: | Ignota |
|---|---|
| Data di carico: | Ignota |
| Nr. Inventario: | Ignoto (Ignoto) |
| Costruttore: | Sconosciuta |
| Materiale: | vetro |
| Dimensioni: | Cilindro D: 13cm, H 17cm |
| Conservazione: | cattivo |
